
1. Introduction: Why Soil pH is the Silent Game-Changer
Imagine walking into your garden and seeing vibrant green leaves, healthy roots, and abundant harvests. The secret often lies beneath your feet—in your soil’s pH balance.
Soil pH, the measure of acidity or alkalinity, plays a critical role in determining how well your plants absorb essential nutrients. If the pH is off, even the richest soil won’t deliver its full potential. In this guide, we’ll explore how to master soil pH for healthier plants and better yields, no matter where you grow.
2. What Is Soil pH and Why It Matters
Soil pH is measured on a scale from 0 to 14:
- Below 7 = acidic soil
- Exactly 7 = neutral soil
- Above 7 = alkaline soil
It’s more than just a number—it directly affects nutrient availability, microbial activity, and root development.
For example, phosphorus, essential for flowering and root growth, becomes less available in highly acidic or alkaline conditions. According to the USDA, the majority of crops grow best between pH 6.0 and 7.0. This range ensures optimal nutrient uptake and healthier plants.
3. Optimal Soil pH Ranges for Different Plants
Different plants thrive at different pH levels. Here’s a quick reference table:
| Crop Type | Optimal pH Range |
|---|---|
| Most Vegetables (tomatoes, beans, cucumbers) | 6.0 – 7.0 |
| Leafy Greens (spinach, lettuce) | 6.0 – 6.8 |
| Acid-Loving Plants (blueberries, azaleas) | 4.5 – 5.5 |
| Root Vegetables (carrots, potatoes) | 5.5 – 6.8 |
| Lawns (cool-season grasses) | 6.3 – 6.8 |
Pro Tip: Before planting, check your crop’s specific pH needs. One-size-fits-all doesn’t work for soil pH.
4. The Consequences of Imbalanced Soil pH
Too Acidic (Low pH)
- Nutrient lock-up: Nitrogen, phosphorus, and potassium become less available.
- Aluminium toxicity can damage roots.
- Stunted growth and lower yields.
Too Alkaline (High pH)
- Micronutrient deficiencies (iron, zinc, manganese).
- Yellowing leaves (chlorosis).
- Reduced water absorption efficiency.
According to the University of Illinois Extension, even a pH shift of 0.5 can significantly affect plant health and harvest quantity.
5. How to Test Your Soil pH
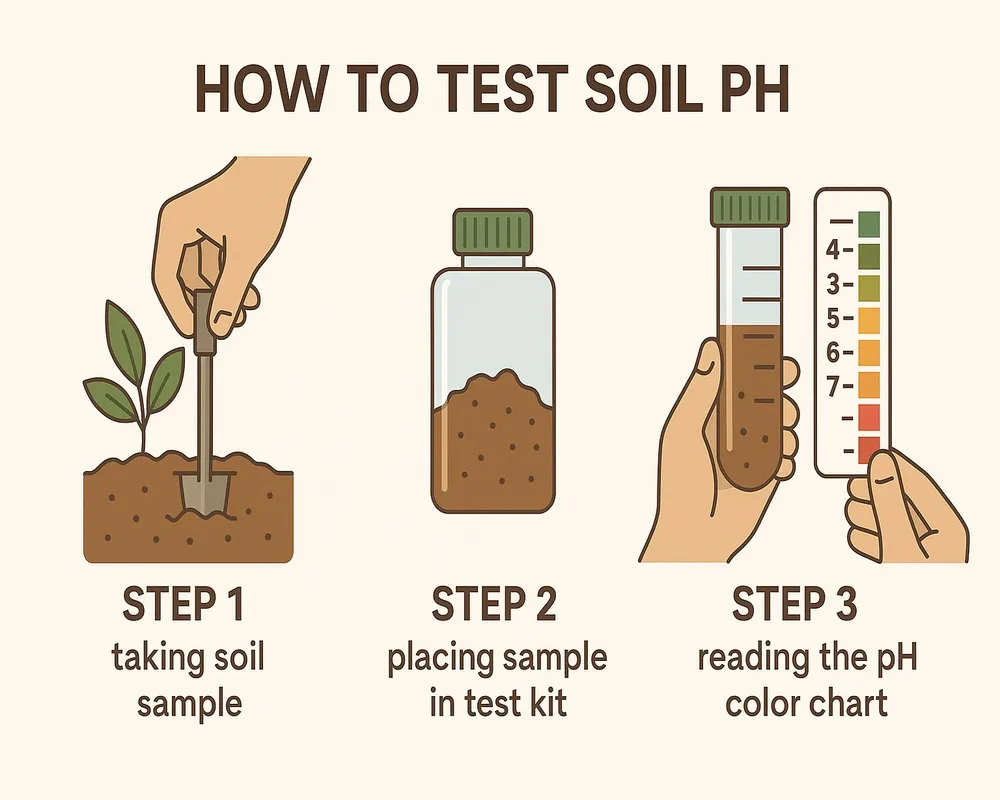
Testing is the first step to mastering soil pH. You have three main options:
- Home Test Kits – Affordable and quick; ideal for routine checks.
- Digital pH Meters – Provide more precise readings.
- Laboratory Soil Tests – Recommended every 2–4 years for detailed results, including nutrient levels and buffering capacity.
DIY Tip: Use the slurry method—mix soil with distilled water and test with a pH meter.
6. How to Adjust Soil pH

If Your Soil is Too Acidic (Low pH)
- Add Agricultural Lime – Raises pH; use finely ground lime for faster results.
- Organic Alternatives – Wood ash, crushed eggshells.
- Apply in fall or early spring for best results.
If Your Soil is Too Alkaline (High pH)
- Apply Elemental Sulfur – Gradually lowers pH over months.
- Use Acidifying Fertilizers – Such as ammonium sulfate.
- Organic Methods – Add peat moss or pine needles.
Important: Always follow recommended application rates based on your soil test.
7. Timing & Best Practices
- Apply Amendments Early – pH adjustments can take weeks or months.
- Incorporate Evenly – Mix amendments into the top 6–8 inches of soil.
- Monitor Annually – Keep records of pH readings and amendments used.
8. Benefits of Balanced Soil pH

- Maximizes nutrient availability.
- Enhances beneficial microbial activity.
- Improves plant resilience against pests and diseases.
- Boosts overall yield quality and quantity.
A 2023 study in Agriculture (MDPI) found that crops grown in optimal pH conditions had up to 25% higher yields compared to those in unbalanced soils.
9. Quick Soil pH Mastery Checklist
- Test your soil’s pH.
- Compare with your crop’s ideal range.
- Apply lime or sulfur as needed.
- Retest after 3–6 months.
- Maintain with regular monitoring.
10. Conclusion
Mastering soil pH is not a one-time task—it’s a continuous process that pays off in healthier plants, richer harvests, and sustainable soil health. By regularly testing, adjusting, and maintaining your soil’s pH, you’re setting the stage for gardening and farming success for years to come.
Further Reading & Resources
- Organic Fertilizer vs Chemical Fertilizer: What Works Better for Your Garden in 2025?
- Best Organic Fertilizers for Tomatoes in 2025: Top Picks & Growing Tips
External Resources (Trusted Agricultural Guides)
- USDA NRCS Soil Health – Official guidelines and tips from the United States Department of Agriculture’s Natural Resources Conservation Service.